Characterizing energy materials
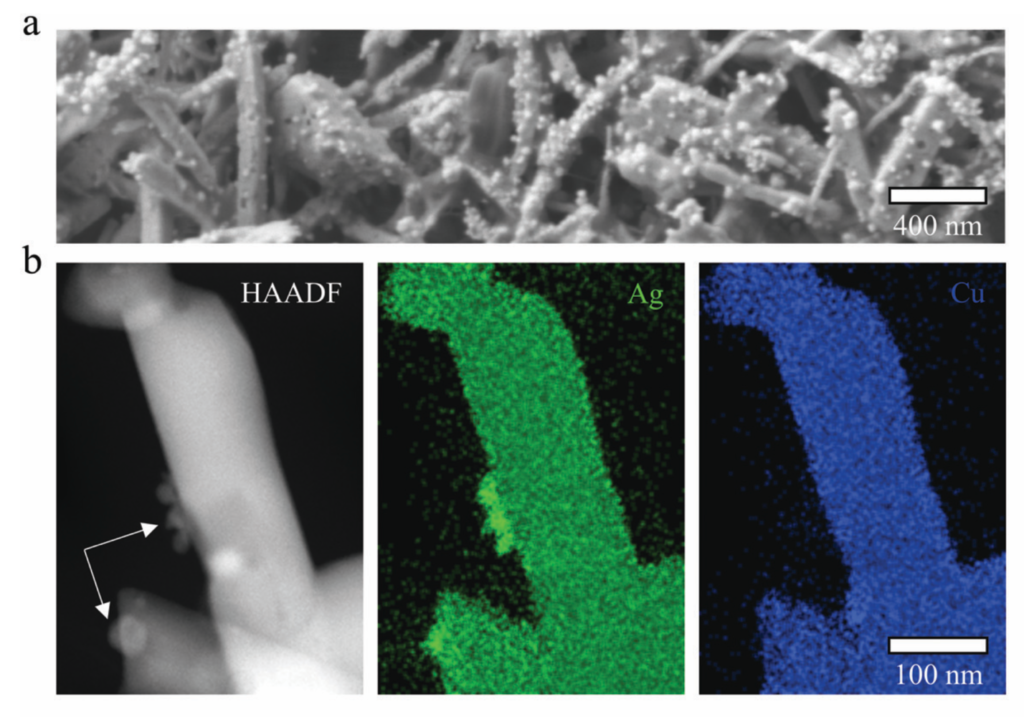 Figure 1 a) SEM image of a post-electrolysis catalyst after being exposed to a constant current density of 400 mA cm2 for 1 h. b) HAADF-STEM image of a single catalyst particle combined with EDX elemental maps of Ag (green) and Cu (blue) The white arrows within the HAADF image point to the in situ formed surface precipitates [2].
Figure 1 a) SEM image of a post-electrolysis catalyst after being exposed to a constant current density of 400 mA cm2 for 1 h. b) HAADF-STEM image of a single catalyst particle combined with EDX elemental maps of Ag (green) and Cu (blue) The white arrows within the HAADF image point to the in situ formed surface precipitates [2].
Energy materials play a crucial role in the storage and conversion of nonrenewable as well as renewable energy, the demand for the latter of which will only grow in the coming years. Electrochemical devices such as fuel cells and electrolyzers will play vital roles in facilitating the transition in the energy and chemical industries, as they enable production of electricity, hydrogen or chemical compounds that are feedstocks for many processes. Often in collaboration with the Helmholtz Institute Erlangen-Nürnberg for Renewable Energy (HI ERN), we study the 3D-structure of electrochemical devices like proton exchange membrane fuel cells (PEMFCs) and electrolyzers on the micro- and near-atomic-scales using FIB/SEM, EDS, and APT. By understanding the structure evolution as a function of electrochemical device operation or different processing routes, it will be possible to identify optimal parameters for e.g. catalyst and pore size distribution, layer thickness etc.. Of particular interest, is studying the surface chemistry and structure of electrocatalysts to gain fundamental understanding, enable improved performance and guide discovery of superior electrocatalysts.
Publications:
- T. Li, O. Kasian, S. Cherevko, S. Zhang, S. Geiger, C. Scheu, P. Felfer, D. Raabe, B. Gault & K. J. J. Mayrhofer. Atomic-scale insights into surface species of electrocatalysts in three dimensions. Nat Catal1, 300–305 (2018). https://doi.org/10.1038/s41929-018-0043-3
- Martić, N., C. Reller, C. Macauley, M. Löffler, A. Reichert, T. Reichbauer, K. Vetter, B. Schmid, D. McLaughlin, P. Leidinger, D. Reinisch, C. Vogl, K. J. J. Mayrhofer, I. Katsounaros, and G. Schmid. “Ag2Cu2O3 – a catalyst template material for selective electroreduction of CO to C2+ products.” (2020). Energy Environ. Sci.. 13, 2993-3006. https://doi.org/10.1039/D0EE01100B
- Martić, N., C. Reller, C. Macauley, M. Löffler, B. Schmid, D. Reinisch, E. Volkova, A. Maltenberger, A. Rucki, K. J. J. Mayrhofer, and G. Schmid. “Paramelaconite-Enriched Copper-Based Material as an Efficient and Robust Catalyst for Electrochemical Carbon Dioxide Reduction.” (2019) Advanced Energy Materials. Vol. 9, 1901228. https://doi.org/10.1002/aenm.201901228